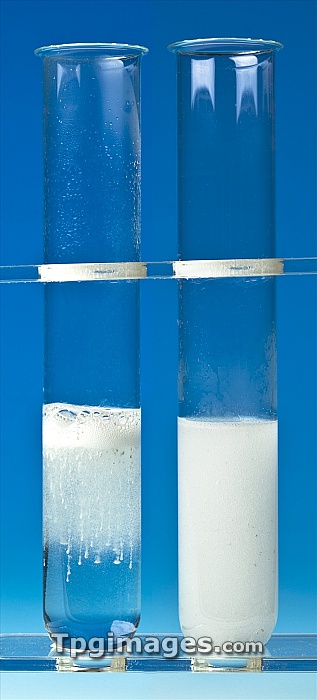
Magnesium reaction with strong and weak acids. Metals (here, magnesium) react more violently with a strong acid (left, hydrochloric acid) than with a weak acid (right, ethanoic acid). The more violent activity (left) includes larger bubbles of hydrogen, which keep the magnesium powder buoyant at the surface. A milky layer descends through the clear acid below, with trails caused by the ascent and descent of individual particles of magnesium. In contrast, the ethanoic acid reaction (right) consists of much smaller bubbles of hydrogen that spread throughout the acid immediately, forming this milky appearance.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP06670813
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
NO
Property Release:
NO
Right to Privacy:
No
Same folder images:

 Loading
Loading