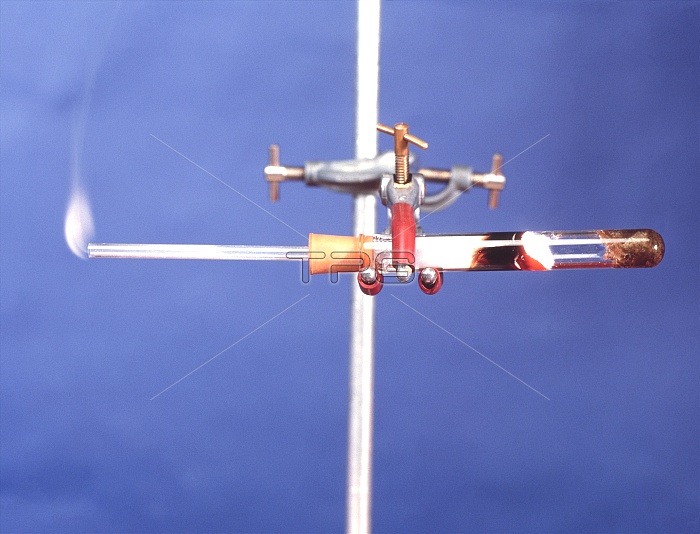
Magnesium reacting with steam. Water (H2O) soaked in mineral wool (far right) is being heated in the test tube. The magnesium (Mg) is reacting violently (black, centre right) with the steam. The reaction is giving off hydrogen gas (H2). The hydrogen gas flows out of the tube (far left), where it has been set alight. Magnesium belongs to group 2 of the periodic table and is a greyish- white, highly reactive metal. The equation for this reaction is: Mg + H2O ---> MgO + H2.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP10163775
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
N/A
Right to Privacy:
No
Same folder images:

 Loading
Loading