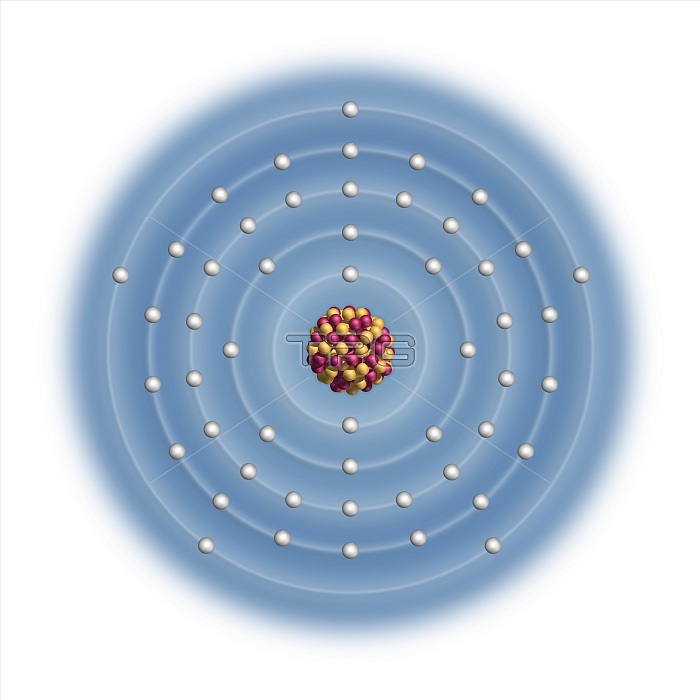
Antimony (Sb). Diagram of the nuclear composition and electron configuration of an atom of antimony-121 (atomic number: 51), the most common isotope of this element. The nucleus consists of 51 protons (red) and 70 neutrons (yellowe). 51 electrons (white) bind to the nucleus, successively occupying available electron shells (rings). The stability of an element's outer electrons determines its chemical and physical properties. Antimony is a metalloid in group 15, period 5, and the p-block of the periodic table. This toxic metal has a melting point of 630 degrees Celsius. It is used in alloys, with its compounds used in fire retardants.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP14864449
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
No
Right to Privacy:
No
Same folder images:

 Loading
Loading