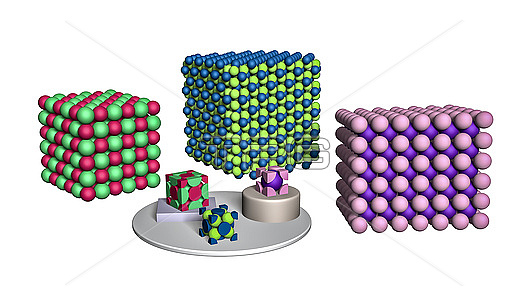
Three ionic crystal forms, illustration. Three of the most common ionic crystal forms are shown here as space filling models. In sodium chloride (NaCl, left), the Na+ (red) and Cl?(mint green) ions form interlocking face-centred cubic structures. Calcium fluoride (CaF2, centre) in the fluorite lattice, has Ca(2+) cations (blue) in a face-centre cubic structure, with fluoride ions (F? yellow-green) in the eight tetrahedral voids. The fluorite lattice is used by Zirconium dioxide (ZrO2), lithium dioxide (Li2O), sodium sulphide (Na2S), strontium fluoride (SrF2) and others. In the caesium chloride lattice (right), chloride anions (Cl? pink) form a primitive cube with a caesium cation (Cs+, purple) in the centre.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP27944657
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
N/A
Right to Privacy:
No
Same folder images:

 Loading
Loading