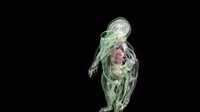
Aluminium metal reacting in a test tube with the liquid halogen element bromine. The reaction is vigorous, producing sparks, flames and thick clouds of aluminium bromide smoke. The equation for the reaction is: 2Al + 3Br2 --> 2AlBr3. Aluminium bromide exists as a dimer, made up of two AlBr3 molecules, so the species is more accurately described as Al2Br6.
Details
WebID:
C01787369
Clip Type:
RM
Super High Res Size:
1920X1080
Duration:
00:00:30.000
Format:
QuickTime
Bit Rate:
25 fps
Available:
download
Comp:
200X112 (0.00 M)
Model Release:
NO
Property Release
No












 Loading
Loading