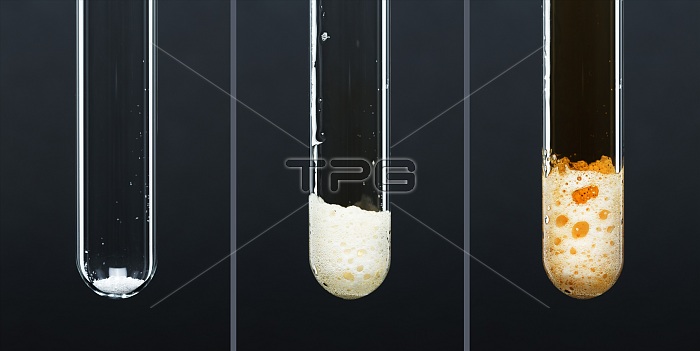
Formation of bromine in a redox reaction. Orange-brown bromine gas (Br2) forms when concentrated (18M) sulfuric acid (H2SO4) is added to white solid potassium bromide (KBr) in a test tube. The initial reaction forms hydrogen bromide gas (HBr), which is then oxidized by the sulfuric acid to form bromine gas (Br2). The two reactions are: KBr + H2SO4 -> HBr + KHSO4 and HBr + H2SO4 -> Br2 + SO2 + H2O. The combined reaction is: KBr + H2SO4 -> KHSO4 + Br2 + SO2 + H2O. The orange-?brown vapour is hydrogen bromide contaminated with the brown colour of bromine.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP22305770
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
No
Right to Privacy:
No
Same folder images:

 Loading
Loading